

Airiver Medical is a clinical stage company developing technologies to help patients who suffer from certain respiratory tract conditions. The company aims to improve the current standard of care for the following conditions:
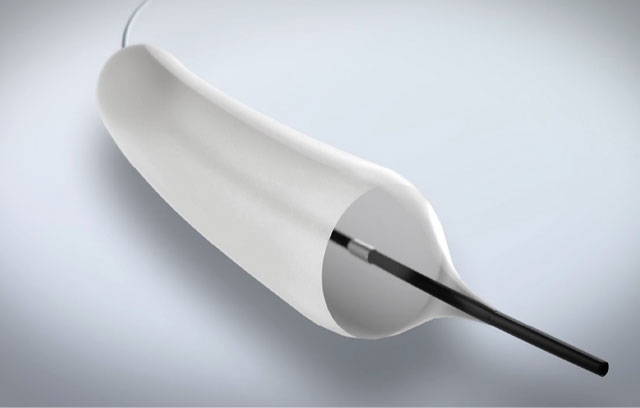
Airiver Medical’s Drug Coated Balloon could offer a new solution for these conditions, potentially creating sustained patency, better outcomes and fewer treatments.
Airiver Medical’s Drug Coated Balloon is an investigational device and is not available for sale in the U.S.
Airway narrowing, often associated with prolonged intubation, tracheostomy, stenting, tuberculosis or transplant. It includes subglottic, tracheal, and bronchial stenosis.
Long-term inflammation of the nasal and paranasal sinus mucosa
Prior experience includes: Boston Scientific, Lutonix, Bard and Philips
Prior experience includes: Covidien, Spectranetics and Philips
Prior experience includes: Entellus Medical and Tactile Medical
Prior experience includes: ACIST Medical Systems, Beckman Coulter and Philips
Prior experience includes: Intersect ENT, Medtronic and Parexel
For any inquiries or questions, please fill out the following form.